BLOG
How to Use Metal Detectors Safely in the Pharmaceutical Industry
The pharmaceutical industry is a critical sector responsible for ensuring the safety and efficacy of medications consumed by millions globally. The integration of metal detectors in pharmaceutical industry practices has emerged as a vital safeguard against contamination, which can compromise product quality and patient safety. According to a recent report by the World Health Organization (WHO), contamination in pharmaceuticals can lead to significantly increased healthcare costs and adverse health effects, emphasizing the necessity for stringent control measures. Recent studies indicate that contaminants, including metallic particles, can enter pharmaceutical products during manufacturing and packaging processes, highlighting the importance of effective detection methods.
Implementing metal detectors within the pharmaceutical production line not only ensures compliance with regulatory standards but also enhances product integrity and consumer trust. Research published in the Journal of Pharmaceutical Sciences indicates that 48% of pharmaceutical recalls are due to contamination issues, underscoring the need for robust measures like metal detection systems. Proper training and usage of metal detectors in the pharmaceutical industry can mitigate risks and prevent costly recalls, thus maintaining the industry's reputation and safeguarding public health. As the industry adapts to emerging challenges and technological advancements, prioritizing the safe use of metal detectors remains crucial for maintaining high standards of pharmaceutical production.

Understanding the Importance of Metal Detection in Pharmaceuticals
In the pharmaceutical industry, ensuring product safety and quality is paramount. Metal detection plays a crucial role in this process, as it helps identify and eliminate potential contaminants that could compromise the integrity of medications. The presence of metal debris—whether from machinery, packaging, or other sources—can lead to severe health risks for consumers and result in costly recalls, regulatory penalties, and damage to a company’s reputation. Consequently, effective metal detection systems are essential for maintaining safety standards throughout production.
Implementing metal detection protocols not only helps to protect consumers but also ensures compliance with stringent industry regulations. By incorporating advanced metal detectors during various stages of pharmaceutical manufacturing, companies can proactively identify contaminants before products reach the market. This proactive approach not only enhances product safety but also instills confidence among healthcare providers and patients. Ultimately, a robust metal detection system is an integral part of the quality assurance strategy in the pharmaceutical sector, reinforcing the industry's commitment to high standards and consumer safety.
Types of Metal Detectors Used in the Pharmaceutical Industry
In the pharmaceutical industry, metal detectors play a crucial role in ensuring product safety and compliance with stringent regulations. Various types of metal detectors are utilized to mitigate risks associated with metal contamination in pharmaceutical products. The most common types include:
1. **Inline Metal Detectors**: These systems are integrated into production lines, allowing for real-time inspection of products as they move through the manufacturing process. They are essential for detecting metallic contaminants before the products reach the packaging stage.
2. **Handheld Metal Detectors**: Portable and versatile, these detectors are often used for spot-checking materials and equipment within the manufacturing environment. They provide quick and immediate results, making them ideal for routine maintenance and inspection tasks.
3. **X-Ray Inspection Systems**: Beyond detecting metal, these sophisticated systems can identify other foreign objects, including glass and stone contaminants. They provide a comprehensive solution to ensure product purity and safety.
**Tips**: When selecting a metal detector for the pharmaceutical industry, consider factors such as sensitivity levels, ease of integration into existing processes, and compliance with regulatory standards. Regular maintenance and calibration are also vital to ensure optimal performance and reliability. Training staff on the proper use of these devices can further enhance the effectiveness of contamination prevention strategies.
Types of Metal Detectors Used in the Pharmaceutical Industry
Safety Protocols When Operating Metal Detectors
When operating metal detectors in the pharmaceutical industry, adhering to strict safety protocols is essential to ensure both product integrity and worker safety. According to a report by the International Society for Pharmaceutical Engineering (ISPE), nearly 80% of pharmaceutical recalls are due to contamination, emphasizing the necessity of effective metal detection systems. The placement of metal detectors at critical control points, such as before packaging and before excipient processing, mitigates risks and enhances compliance with regulatory standards, including those set by the FDA.
Training personnel in the proper operation and maintenance of metal detectors is crucial. OSHA guidelines recommend that all employees involved in the usage of such equipment should undergo regular training. This not only minimizes the risk of malfunction but also ensures that the team can identify and troubleshoot issues swiftly, adhering to the Good Manufacturing Practices (GMP) outlined in pharmaceutical manufacturing. Additionally, routine calibration and performance verification of metal detectors are vital, as studies indicate that even slight deviations can lead to undetected foreign contaminants, risking patient safety. Thus, implementing these safety protocols not only protects the product but also upholds the company's reputation within the industry.
How to Use Metal Detectors Safely in the Pharmaceutical Industry - Safety Protocols When Operating Metal Detectors
| Protocol | Description | Frequency of Review | Responsible Personnel |
|---|---|---|---|
| Training | All operators must complete a training program on using metal detectors and interpreting results. | Annually | Safety Officer |
| Equipment Calibration | Regular calibration of metal detectors to ensure accuracy. | Monthly | Maintenance Technician |
| Environmental Checks | Ensure that the operating environment is conducive for metal detection. | Daily | Floor Supervisor |
| Reporting Procedures | Document any incidents or findings during metal detection processes. | As needed | Quality Control Staff |
| Personal Protective Equipment (PPE) | Ensure operators wear necessary PPE while conducting checks. | Ongoing | All Operators |
Best Practices for Metal Detection Procedures
In the pharmaceutical industry, implementing best practices for metal detection is crucial to ensure product safety and maintain compliance with stringent regulations. One key practice is conducting regular calibration of metal detectors.
Regular calibration ensures the equipment is functioning optimally and can accurately identify foreign metal contaminants. This involves using test pieces of different metals and sizes to validate the detector’s sensitivity and performance.
Another essential practice is integrating metal detection into the production workflow. This includes positioning metal detectors at critical control points, such as before packaging and after processing. Staff should be trained to operate the metal detectors effectively and understand the procedures for responding to detections.
Additionally, maintaining detailed records of inspections and detections can greatly enhance traceability and accountability, which are vital in the pharmaceutical sector.
This helps mitigate risks associated with metal contamination, thus protecting both consumers and brand integrity.
Training and Compliance for Staff in Metal Detection Safety
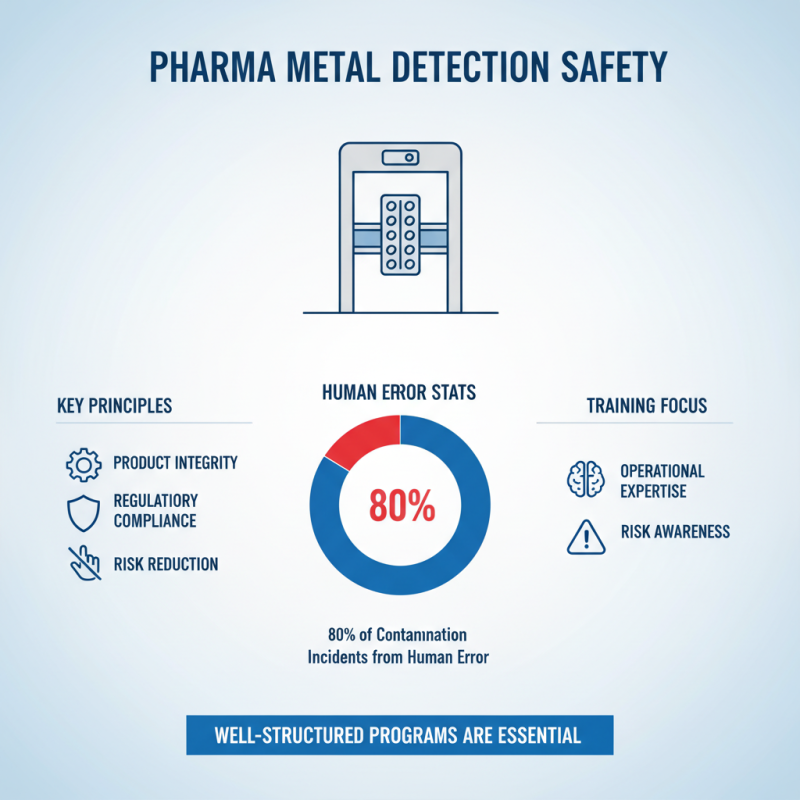
In the pharmaceutical industry, ensuring safety during metal detection processes is crucial to maintain product integrity and compliance with regulatory standards. Employees should undergo comprehensive training that covers not only the operational aspects of metal detectors but also the potential risks associated with improper use. According to a report by the Pharmaceutical Industry Standards Association, 80% of contamination incidents can be traced back to human error, underscoring the need for well-structured training programs.
Effective metal detection training should include hands-on sessions that familiarize staff with the equipment, as well as regular assessments to reinforce best practices. An industry survey found that companies implementing ongoing training saw a 30% decrease in contamination events. Additionally, emphasizing compliance with regulatory mandates, such as those set by the FDA and EMA, is vital. Employees must understand the critical role they play in upholding these standards, enhancing both their competence and the overall safety of pharmaceutical products.
Related Posts
-

2025 How to Choose the Best Varpe Checkweigher for Your Business Needs
-

Top 5 Benefits of Xray Inspection for Quality Control and Safety Standards
-
Top 10 Factors Influencing Food X-Ray Machine Prices You Must Know
-
Top 5 Food Inspection Systems Revolutionizing Quality Control in the Industry
-

Top 10 Food X-Ray Machine Prices and Insights for 2023: A Comprehensive Guide
-

How to Use X-Ray Technology in the Food Industry for Enhanced Safety and Quality
